Chemotherapy-Induced Cardiotoxicity: Mechanisms, Detection and Emerging Therapies in Cardio-Oncology
DISCOVERIES (ISSN 2359-7232), 2025, volume 13
ORIGINAL ARTICLE
FULL text (PDF)
CITATION: Sánchez B, González P, Goveo I, Contreras P, Dominguez L, Manrique P, Vargas M. Chemotherapy-Induced Cardiotoxicity: Mechanisms, Detection and Emerging Therapies in Cardio-Oncology. Discoveries 2025, 13(4): e217. DOI: 10.15190/d.2025.16
Chemotherapy-Induced Cardiotoxicity: Mechanisms, Detection and Emerging Therapies in Cardio-Oncology
Brandon Sánchez 1,*, Pamela González 1, Iván Goveo 3, Pedro Contreras 2, Luis Domínguez 2, Paola Manrique 3, Manuel Vargas 1
- 1 Pontificia Universidad Católica Madre y Maestra. Santiago, Dominican Republic
- 2 Corazones del Cibao, Santiago, Dominican Republic
- 3 Universidad Autonoma de Guadalajara, Guadalajara, Mexico
* Corresponding authors: Brandon Sánchez, Pontificia Universidad Católica Madre y Maestra, Santiago de los Caballeros, Republica Dominicana; email: brandonsanchezrdgz@gmail.com; ORCID: 0009-0000-4525-7975.
Abstract
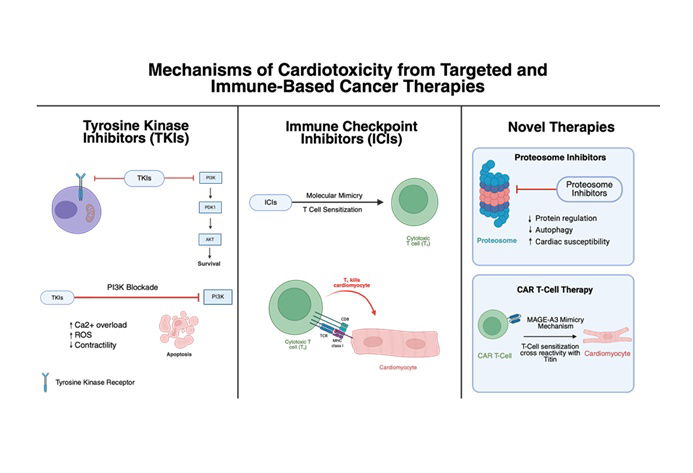
Cancer remains a leading cause of global mortality, with annual incidence projected to exceed 35 million cases by 2050. Modern antineoplastic therapies have improved survival outcomes at the risk of increasingly associated cardiovascular complications, collectively termed cancer therapy related cardiac dysfunction (CTRCD). Anthracyclines and HER2-targeted therapies remain the most well-characterized cardiotoxic agents. Anthracyclines cause irreversible, dose-dependent myocardial injury through mechanisms including oxidative stress, iron dysregulation, mitochondrial dysfunction, and topoisomerase IIβ inhibition, leading to progressive ventricular dysfunction and heart failure. HER2-directed therapies, such as trastuzumab, interfere with cardioprotective ErbB signaling, typically producing reversible cardiac impairment. Other oncologic treatments - including tyrosine kinase inhibitors, VEGF antagonists, and immune checkpoint inhibitors - contribute to hypertension, ischemic injury, and immune-mediated myocarditis. Newer modalities, such as proteasome inhibitors, histone deacetylase inhibitors, and CAR T-cell therapy, have expanded the spectrum of treatment-associated cardiotoxicity. Early CTRCD detection through multimodal strategies—including echocardiographic assessment with global longitudinal strain, cardiac magnetic resonance imaging, and serial measurement of troponins and natriuretic peptides—facilitates timely intervention. Risk stratification tools such as the HFA–ICOS score enable personalized monitoring and therapeutic planning. Preventive and management strategies incorporate cardioprotective agents like ACE inhibitors, β-blockers, dexrazoxane, and emerging therapies such as SGLT2 inhibitors. Modern cardio-oncology emphasizes a multidisciplinary, precision-based approach integrating early detection, genetic risk assessment, and targeted prophylaxis to preserve cardiac function while maintaining oncologic efficacy, thereby enhancing both survival and quality of life for cancer patients.